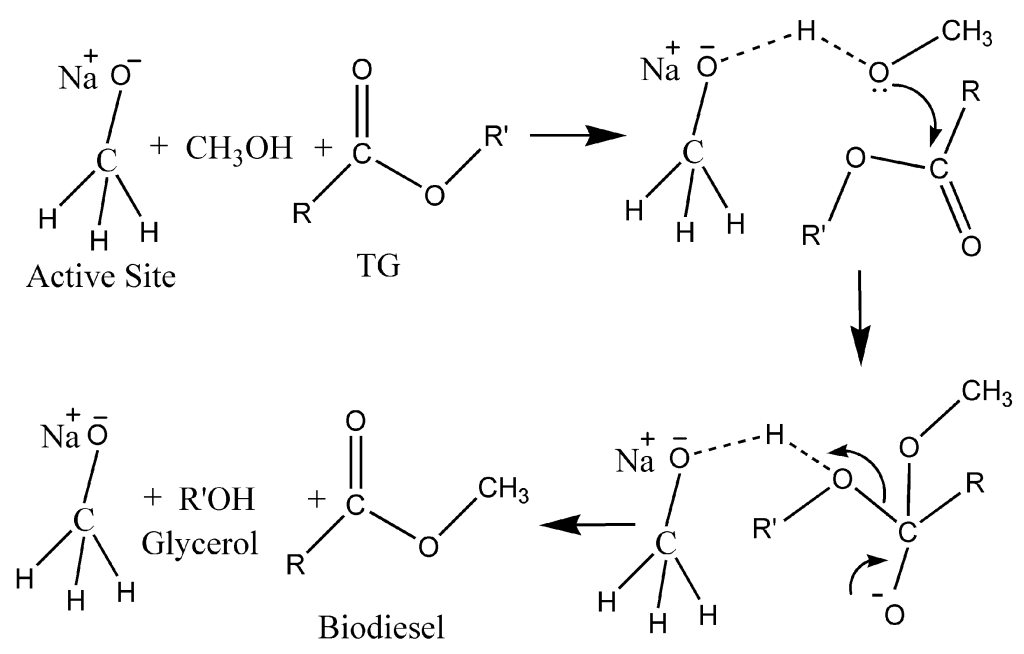
Figure 1Plausible homogeneous catalytic mechanism of transesterification of WCO using NaOMe/zeolite[1]
- Brønsted Base Properties:
The high basicity of sodium methoxide is primarily due to the ability of the methoxide anion (CH3O-) to readily accept a proton, forming methanol (CH3OH) and the hydroxide ion (OH-).[12] This proton transfer reaction can be represented as follows:
CH3O- + H+ → CH3OH
The methoxide anion is a strong base, with a pKa value around 15-16, making it a potent proton acceptor1. This Brønsted base reactivity is the foundation for many of the synthetic applications of sodium methoxide, as it can be used to deprotonate acidic protons on a wide range of organic substrates.[[2]]
When sodium methoxide is dissolved in protic solvents like water or alcohols, the methoxide anion can undergo this proton transfer equilibrium, generating the corresponding alcohol (e.g., methanol) and a hydroxide ion. This equilibrium can be influenced by factors such as pH and the presence of other species in the solution.
- Nucleophilic Reactivity:
In addition to its Brønsted base properties, sodium methoxide can also act as a nucleophile, with the methoxide group (CH3O-) attacking electrophilic carbon centers 12.[3] This nucleophilic reactivity is particularly useful in various organic reactions, such as alkylation and esterification.
In alkylation reactions, the methoxide anion can displace a leaving group (e.g., a halide) from an alkyl halide, forming a new carbon-oxygen bond and producing an ether. This substitution reaction can be represented as follows:
R-X + CH3O- → R-O-CH3 + X-
where R represents an alkyl group and X is the leaving group (e.g., a halide).
In esterification reactions, the methoxide anion can act as a nucleophile, attacking the carbonyl carbon of an acyl halide or an anhydride, displacing the leaving group and forming a new ester bond. This process can be depicted as:
R-C(O)-X + CH3O- → R-C(O)-O-CH3 + X-
where R represents an alkyl or aryl group, and X is the leaving group (e.g., a halide or alkoxide).
The dual reactivity of sodium methoxide, both as a Brønsted base and as a nucleophile, makes it a versatile and valuable reagent in organic synthesis. The ability to deprotonate acidic protons and to undergo substitution or addition reactions with electrophilic centers allows for a wide range of synthetic transformations, including alkylations, esterifications, and other important organic reactions.[4], [5]
Reference:
1. Argaw Shiferaw K, M.J., Yu E, Choi E-Y, Tarte NH. Sodium Methoxide/Zeolite-Supported Catalyst for Transesterification of Soybean Waste Cooking Oil for Biodiesel Production. Inorganics. 2023; 11(4):163. https://doi.org/10.3390/inorganics11040163, Sodium Methoxide/Zeolite-Supported Catalyst for Transesterification of Soybean Waste Cooking Oil for Biodiesel Production. 2023.
2. LINDSAY, A.M.-G.S.o.B.A.a.B.O.D.d., Durham University). Mechanism-Guided Studies of Brønsted Acid and Base Organocatalysis. (2010).
3. Terrier, F.M.n.a.s.J.W.S., Modern nucleophilic aromatic substitution. (2013).
4. Clayden, J., Greeves, N., & Warren, S. (2012). Organic chemistry. Oxford University Press, USA., Organic chemistry. (2012).
5. Johnson, S., Meyers, M., Hyme, S., & Leontyev, A. (2019). Green chemistry coverage in organic chemistry textbooks. Journal of Chemical Education, 97(2), 383-389., Green chemistry coverage in organic chemistry textbooks. (2019).
